If there are superstars in battery research, you would be safe in identifying at least one of them as Yi Cui, a scientist at Stanford University, whose research group over the years has introduced some key breakthroughs in battery technology.
Now Cui and his research team, in collaboration with SLAC National Accelerator Laboratory, have offered some exciting new capabilities for lithium-ion batteries based around a new polymer material they are using in the current collectors for them. The researchers claim this new design to current collectors increases efficiency in Li-ion batteries and reduces the risks of fires associated with these batteries.
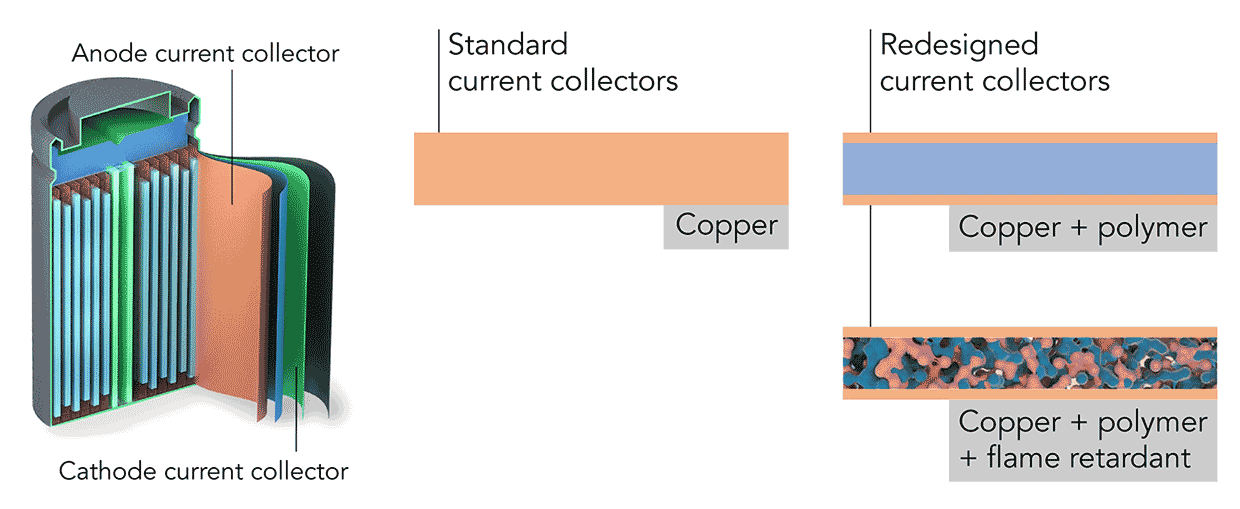
Current collectors are thin metal foils that distribute current to and from electrodes in batteries. Typically these metal foils are made from copper. Cui and his team redesigned these current collectors so that they are still largely made from copper but are now surrounded by a polymer.
The Stanford team claim in their research published in the journal Nature Energy that the polymer makes the current collector 80 percent lighter, leading to an increase in energy density from 16 to 26 percent. This is a significant boost over the average yearly increase of energy density for Li-ion batteries, which has been stuck at 5 percent a year seemingly forever.
This method of lightening the batteries is a bit of a novel approach to boosting energy density. Over the years we have seen many attempts to increase energy density by enlarging the surface area of electrodes through the use of new electrode materials—such as nanostructured silicon in place of activated carbon. While increased surface area may increase charge capacity, energy density is calculated by the total energy over the total weight of the battery.
The Stanford team have calculated the increase of 16 to 26 percent in the gravimetric energy density of their batteries by replacing the commercial copper/aluminum current collectors (8.06 mg/cm2 for copper and 5.0 mg/cm2 for aluminum) with their polymer collections current collectors (1.54 mg/cm2 for polymer-copper material and 1.05 mg/cm2 for polymer-aluminum).
“Current collectors don’t contribute to the total energy but contribute to the total weight of battery,” explained Yusheng Ye, a researcher at Stanford and co-author of this research. “That’s why we call current collectors ‘dead weight’ in batteries, in contrast to ‘active weight’ of electrode materials.”
By reducing the weight of the current collector, the energy density can be increased, even when the total energy of the battery is almost unchanged. Despite the increased energy density offered by this research, it may not entirely alleviate so-called “range anxiety” associated with electric vehicles in which people have a fear of running out of power before reaching the next charge location. While the press release claims that this work will extend the range of electric vehicles, Ye noted that the specific energy improvement in this latest development is based on the battery itself. As a result, it is only likely to have around a 10% improvement in the range of an electric vehicle.
“In order to improve the range from 400 miles to 600 miles, for example, more engineering work would need to be done taking into account the active parts of the batteries will need to be addressed together with our ultra-light current collectors,” said Ye.
Beyond improved energy density efficiency, the polymer-based charge collectors are expected to help reduce the fires associated with Li-ion batteries. Of course, traditional copper current collectors don’t contribute to battery combustion on their own. The combustion issues in Li-ion batteries are related to the electrolyte and separator that are not used within the recommended temperatures and voltage windows.
“One of the key innovations in our novel current collector is that we are able to embed fire retardant inside without sacrificing the energy density and mechanical strength of the current collector,” said Ye. “Whenever the battery has combustion issues, our current collector will instantaneously release the fire retardant and extinguish the fire. Such function cannot be achieved with traditional copper or aluminum current collector.”
The researchers have patented the technology and are in discussions with battery manufacturers for commercialization. Cui and his team have already worked out some of the costs associated with adopting the polymer and they appear attractive. According to Ye, the cost of the polymer composite charge collector is around $1.3 per m2, which is a bit lower than the cost of copper foil, which is around $1.4 per m2. With these encouraging numbers, Ye added: “We are expecting industry to adopt this technology within the next few years.”